
Nov . 05, 2024 05:11
Back to list
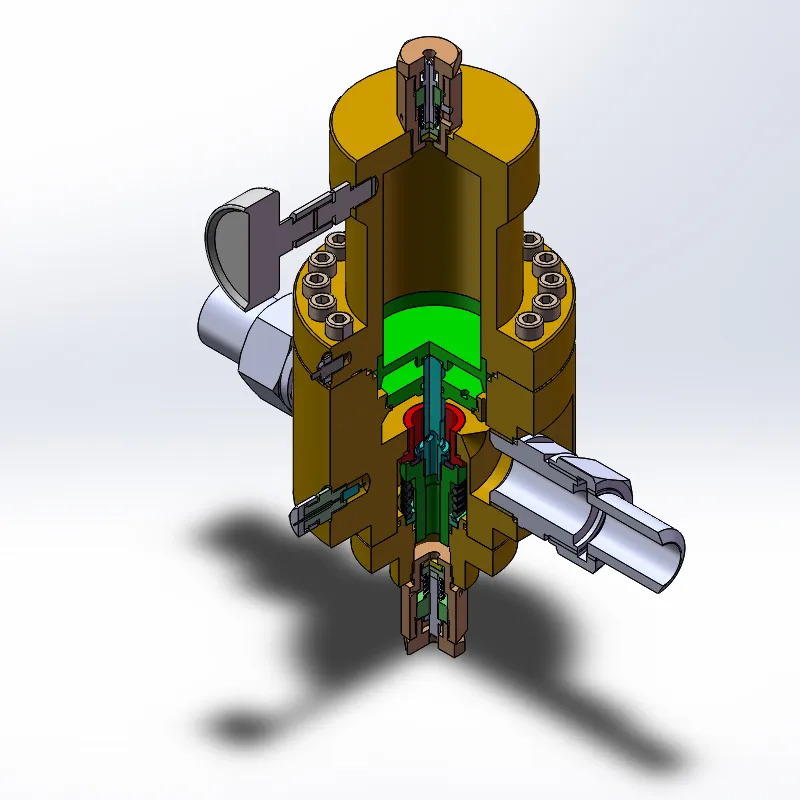
منظم الغاز
The Organization of Gases Understanding Their Behavior and Applications
Gases play a vital role in our daily lives and the functioning of various natural and industrial processes. Understanding the behavior of gases, particularly their organization, is crucial for fields ranging from chemistry and physics to environmental science and engineering. This article explores the fundamental principles that govern the organization of gases, their characteristics, and practical applications.
.
Boyle’s Law states that at a constant temperature, the pressure of a gas decreases as its volume increases. This principle explains why a balloon expands when released from a tighter space; the decrease in surrounding pressure allows the gas inside to expand. Charles’s Law, on the other hand, illustrates the relationship between temperature and volume; when the temperature of a gas increases, its volume increases as well, provided the pressure remains constant. These laws are fundamental for understanding phenomena in both everyday life and industrial processes.
منظم الغاز
In addition to these laws, the ideal gas equation (PV=nRT) integrates pressure (P), volume (V), the number of moles of gas (n), and temperature (T) to describe the state of an ideal gas. While real gases may not always behave ideally due to intermolecular forces or high pressures, this equation serves as a useful model for many applications.
The organization of gases is not just a theoretical concept; it has practical implications as well. In the energy sector, for instance, the understanding of gas behavior is essential for the efficient design of engines and turbines. Combustion engines rely on the principles of gas expansion and pressure to convert fuel into kinetic energy. Similarly, in the field of environmental science, controlling and predicting gas emissions from various sources is crucial for mitigating climate change and ensuring air quality.
Moreover, the organization of gases extends to industrial applications. For example, in the production of chemicals, gases are often reacted under controlled conditions to optimize yield. The food industry also utilizes gases in packaging to preserve freshness and enhance shelf life by altering the composition of the atmosphere inside packages.
In conclusion, the organization of gases is a fundamental aspect of both nature and technology. By understanding the principles governing gas behavior, we can harness their properties for various applications, leading to advancements in science, industry, and everyday life. Whether it's through the design of better engines, the improvement of air quality, or the innovation of food preservation techniques, the study of gases remains an essential element of progress in our world.
Next:
Latest news
-
Safety Valve Spring-Loaded Design Overpressure ProtectionNewsJul.25,2025
-
Precision Voltage Regulator AC5 Accuracy Grade PerformanceNewsJul.25,2025
-
Natural Gas Pressure Regulating Skid Industrial Pipeline ApplicationsNewsJul.25,2025
-
Natural Gas Filter Stainless Steel Mesh Element DesignNewsJul.25,2025
-
Gas Pressure Regulator Valve Direct-Acting Spring-Loaded DesignNewsJul.25,2025
-
Decompression Equipment Multi-Stage Heat Exchange System DesignNewsJul.25,2025

